
LCP Protocols >> Manual LCP Crystallization
Manual LCP Crystallization
LCP crystallization trials can be set up manually or robotically. Protocols for manual crystallization are provided in this section; using robotics for LCP crystallization is extensively discussed in Cherezov et al., 2004; Cherezov and Caffrey, 2007. It is preferable to perform LCP crystallization trials in glass sandwich plates, described in Cherezov and Caffrey, 2003; Cherezov et al., 2004. These plates have excellent optical properties for detection of extremely small colorless protein crystals growing in an LCP matrix. Glass sandwich plates can be purchased from Paul Marienfeld GmbH or Molecular Dimensions or can be assembled from separately ordered glass slides and perforated spacers. Alternatively, most commercial micro-batch, sitting or hanging drop plates could be used for setting up manual crystallization trials with the caveat that they may provide less than optimal conditions for detection of small colorless crystals, primarily due to the scattering of light from a rough boundary between the LCP bolus and a precipitant solution (Fig. 1A-C). The problem can be circumvented by sandwiching the LCP bolus with a 5 mm diameter glass coverslip (Warner Instruments, cat.# W2 64-0700) as shown in Fig. 1D,E and was used by Lunde et al., 2006. Crystallization setup starts with mixing protein solution with lipid as described in Reconstitution of Protein in LCP. It is advisable to have plates and screening solutions ready before starting protein reconstitution in LCP, and to proceed to crystallization setup immediately after forming protein-laden LCP, because some proteins may not be stable in LCP without added precipitant solution. The whole process of manual setting up a 96-well plate including mixing of protein and lipid takes about 1 hr.

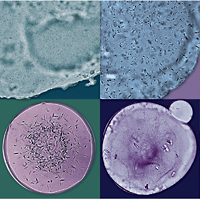
Figure 1. Different ways of setting up crystallization trials in LCP: A. Microbatch, B. Sitting drop, C. Hanging drop, D. Modified hanging drop, E. Modified microbatch. In (D and E) LCP bolus (black) is sandwiched using a small 5 mm in diameter glass coverslip in order to improve optical properties and facilitate detection of crystals growing in LCP. The best conditions for detecting small colorless crystals are achieved when crystallization trials are performed in glass sandwich plates. (F – H) show top views of the glass sandwich plates used in our laboratory. Plates (F and G) are based on a standard microscope slide, which are convenient to use for setting up crystallization trials manually. Plate (H) has an SBS-complaint footprint and is suitable for both robotic and manual setups.
MATERIALS:
- Protein reconstituted in LCP prepared as described in Reconstitution of Protein in LCP protocol.
- 10 μL gas-tight removable needle syringe without a needle (Hamilton, cat.# 7653-01).
- Repetitive syringe dispenser (Hamilton, cat.# 83700) (The Hamilton syringe dispenser can be modified as described in Cherezov & Caffrey, 2005 to decrease the dispensing volume ~3 times, from 200 nL to 70 nL).
- Short (0.375 inch), flat-tipped needle (point style 3, gauge 26, Hamilton, cat.# 7804-03).
- Glass sandwich plate (Paul Marienfeld GmbH, cat# 0890003; or Molecular Dimensions, cat# MD11-55).
- Square glass coverslips, 18 mm (Fisher).
- Crystallization screens (Note 1: It is convenient to use commercial sparse matrix screens for initial screening. On average, however, about 30 % of commercial screen conditions are not compatible with LCP crystallization, transforming LCP into lamellar or hexagonal phase or completely dissolving the lipid (Cherezov et al., 2001). Such conditions can be diluted 2 times with water to increase their compatibility. Additionally, more specific grid or sparse matrix screens can be prepared following results prescreening assays such as LCP-FRAP (Cherezov et al., 2008) or LCP-Tm (Liu et al., submitted). Note 2: When protein is crystallized in a complex with its ligand it may be necessary to supplement crystallization screens with the ligand. During LCP crystallization the soluble fraction of LCP is diluted ~50 times due to the vast excess of precipitant (1 μL) over the LCP bolus (50 nL), thus significantly depleting concentration of the ligand in the vicinity of the protein).
PROCEDURE:
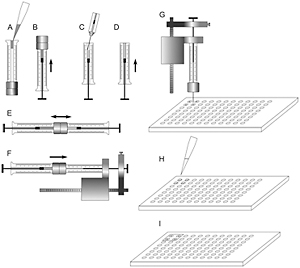
Figure 2. A sequence of steps to prepare LCP and set up crystallization trials in a glass sandwich plate.
- Attach a 10 μL gas-tight syringe to a repetitive syringe dispenser.
- Transfer protein-laden LCP into the 10 μL syringe affixed to the repetitive dispenser (Fig. 2F).
- Attach a short removable needle to the 10 μL syringe.
- Push the plunger until the cubic phase starts coming out from the needle. Fix the plunger with a gripping nut of the repetitive dispenser.
- Place a glass sandwich plate on a flat surface.
- Position the syringe needle at the center of the first well ~200-300 μm above the surface and press on the button of the dispenser to deliver 200 nL of the protein laden cubic phase bolus (Fig. 2G) (If the needle is too far from the glass surface the LCP bolus coming out of the needle as a tube curls back and sticks to the needle. If the needle is too close to the glass surface the LCP bolus balls up and sticks to the needle. Both of these cases result in no or incomplete delivery. The right distance and feeling for accurate dispensing come with practice and are relatively easy to achieve).
- Repeat Step 5 to dispense LCP into three more wells forming a 2x2 square.
- Add 1 μL of precipitant solutions on top of the LCP boluses in each of the four wells (Fig. 2H).
- Cap the four loaded wells with a 18 mm square glass coverslip (Fig. 2I). Use a wooden toothpick to press on the coverslip around the wells to properly seal them.
- Repeat Steps 5 – 8 until the whole plate is filled up.
- Incubate the plate at 20°C (Crystallization plates can be incubated at different temperatures, however, the monoolein-based LCP is stable only at temperatures above 17°C (Qiu and Caffrey, 2000). Recently, several new lipids were developed specifically for the low temperature LCP crystallization (Misquitta et al., 2004; Yamashita et al., 2008). It is important to avoid temperature fluctuations during plate incubation and imaging, because fluctuations by just few degrees can induce formation of liquid droplets inside the cubic phase. These droplets scatter light, making crystal detection more difficult).
For additional details see Caffrey and Cherezov, 2009.
REFERENCES:
Caffrey, M., and V. Cherezov. (2009) Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4: 706-731. >>
Cherezov, V., Fersi, H., and M. Caffrey. (2001) Crystallization screens: compatibility with the lipidic cubic phase for in meso crystallization of membrane proteins. Biophys. J. 81: 225-242. >>
Cherezov, V., and M. Caffrey. (2003) Nano-volume plates with excellent optical properties for fast, inexpensive crystallization screening of membrane proteins. J. Appl. Cryst. 36: 1372-1377. >>
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y.F., and M. Caffrey. (2004) A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallogr. D Biol. Crystallogr. 60: 1795-1807. >>
Cherezov, V., and Caffrey, M. (2005) A simple and inexpensive nanoliter-volume dispenser for highly viscous materials used in membrane protein crystallization. J. Appl. Cryst. 38: 398-400. >>
Cherezov, V., and M. Caffrey. (2007) Miniaturization and automation for high-throughput membrane protein crystallization in lipidic mesophases. In: Chayen NE, ed. Protein crystallization strategies for structural genomics, International University Line, San Diego. >>
Lunde, C.S., Rouhani, S., Facciotti, M.T., and R.M. Glaeser. (2006) Membrane-protein stability in a phospholipid-based crystallization medium. J. Struct. Biol. 154: 223-231. >>
Misquitta, Y., Cherezov, V., Havas, F., Patterson, S., Mohan, J.M., Wells, A.J., Hart, D.J., and M. Caffrey. (2004) Rational design of lipid for membrane protein crystallization. J. Struct. Biol. 148, 169-175. >>
Qiu, H., and M. Caffrey. (2000) The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials 21: 223-34. >>
Yamashita, J., Shiono, M., and M. Hato. (2008) New Lipid Family That Forms Inverted Cubic Phases in Equilibrium with Excess Water: Molecular Structure - Aqueous Phase Structure Relationship for Lipids with 5,9,13,17-Tetramethyloctadecyl and 5,9,13,17-Tetramethyloctadecanoyl Chains. J. Phys. Chem. B 112, 12286-12296. >>
CONTACT US: USC | Cherezov Lab | cherezov@usc.edu