

Box1.
LCP Properties:
- Transparent
- Optically isotropic
- Viscous and sticky
- Gel-like
- Large surface area/volume
- Single lipid bilayer
Box 2.
LCP applications:
- Drug delivery
- Sustained release
- Topical application
- Uptake of contaminants
- Food emulsifier
- Biosensors
- Separation of biomolecules
- Chemical synthesis
- Protein crystallization
Introduction

Figure 1. Temperature-composition phase diagram of monoolein, the most common lipid for LCP crystallization. The phase diagram represents a metastable state at temperatures below 20 C. Re-drawn from Briggs & Caffrey, 1996.

Figure 2. Cartoon pictures of three bicontinuous LCPs with different space groups. Two networks of non-intersecting water channels are shown in different colors.
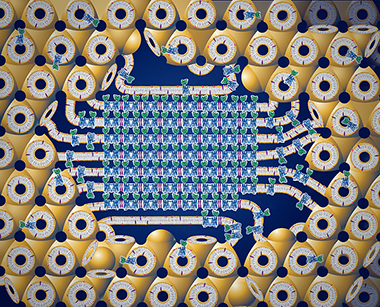
Lipidic cubic phase (LCP) is one of many liquid crystalline phases that form spontaneously upon mixing lipids with water at proper conditions (Fig 1). Structuraly LCP consists of a single lipid bilayer that follows an infinite periodic minimal surface (IPMS) dividing the space into two non-intersecting networks of water channels. There are three common types of bicontinuous LCPs with different space group symmetry (Fig. 2). LCP possesses many unique properties (Box 1), making it an attractive tool for a number of applications (Box 2). Crystallization of membrane proteins in LCP was introduced in 1996 by Landau & Rosenbusch (1). This technique has proven to be crucial for elucidating structural mechanisms of action of several microbial rhodopsins, as well as provided the first high-resolution details of human G protein-coupled receptors (GPCR) bound to diffusible ligands. Success of using LCP for growing highly ordered crystals of challenging human membrane proteins can be attributed to at least two factors. LCP provides a more native-like membrane environment for proteins as opposed to a rather harsh environment associated with detergent micelles. Crystals grown in LCP have type I packing with protein molecules making contacts not only through hydrophilic but also through hydrophobic portions resulting in lower solvent content and better crystal ordering (Fig. 3). Click here >> to see the stats on membrane protein structures crystallized in LCP.
References
1. Landau, E.M., and J.P. Rosenbusch. (1996) Lipidic cubic phases: a novel concept for the crystallization of membrane proteins.
Proc. Natl. Acad. Sci. U S A 93: 14532-14535. >>
Figure 3. Cartoon representation of the in meso crystallization process. Integral membrane protein molecules (blue-green) are initially embedded into the lipid bilayer of the LCP (yellow), which provides a connectivity for the 3D diffusion. Addition of a precipitant induces a crystal nucleation. The crystal (in the center) is attached to the bulk LCP through a multilamellar lipid portal, which feeds the growing crystal with the protein molecules.
CONTACT US: USC | Cherezov Lab | cherezov@usc.edu