
LCP Protocols >> Harvesting Crystals from LCP
Harvesting crystals from LCP
When crystals are grown in a glass sandwich plate, opening an individual well for crystal harvesting presents a challenge. Due to a strong adhesion of the double sticky spacer to the glass slides it is impossible to just peel off the cover slip to expose a single well for crystal harvesting. Therefore a piece of the cover glass should be cut out and removed first. How to do this is described in Section 1 of the Procedures. If crystals are obtained in commercial plastic trays, in which opening a well is a trivial procedure, skip Section 1 and proceed directly to the crystal harvesting section 2.
MATERIALS:
- Plate with protein crystals.
- Precipitant solution for the condition that supports crystal growth.
- Stereo zoom microscope with a linear rotating polarizer and analyzer (we use Nikon SMZ-1500).
- Capillary cutting stone (Hampton Research, cat# HR4-334), or a tungsten-carbid glass cutter (Hampton Research, cat# HR4-469).
- Fine point tweezers (Ted Pella, cat# 510).
- Needle with a sharp point angled at 45 degrees (Ted Pella, cat# 13650)
- Adjustable pipette 0.1-10 μL with disposable tips for transferring the precipitant solution.
- Magnetic CrystalWand (Hampton Research, cat# HR4-729).
- Low profile dewar (Hampton Research, cat# HR5-102, or HR4-673).
- Magnetic CrystalCaps, ALS style for compatibility with automounters (Hampton Research, cat# HR4-779).
- MiTeGen MicroMounts (cat# M2-Lxx-yy; we prefer MiTeGen MicroMounts over standard nylon loops for harvesting crystals from LCP due to their rigidity and thinner profile, which allow for easier penetration into a stiff LCP gel and for dragging a minimal amount of lipids along with the crystal).
- ALS-style pucks (Boyd Technologies) or Uni-pack (MiTeGen).
PROCEDURES:
1. Opening a well in the glass sandwich plate
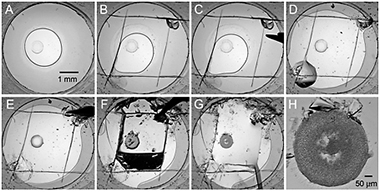
Figure 1. Sequence of steps illustrating opening of an individual well in a glass sandwich plate for crystal harvesting.
- Place a glass sandwich plate with crystals on a harvesting stereo microscope with a variable zoom.
- Focus the microscope on a well with crystals, using a low zoom, so that the whole well is visible in the field of view (Fig. 1A).
- Score the cover glass in four strokes forming a square inside the well boundaries using a sharp corner of a Hampton Research ceramic capillary cutting stone (Fig. 1B).
- Using a strong sharp point tweezers press around the scored perimeter to propagate the scratches through the thickness of the cover glass (Fig. 1C).
- Punch two small holes in the cover glass at opposite corners of the square.
- Inject 2-3 μL of precipitant solution into the well through one of the holes to reduce dehydration during the subsequent well opening steps (Fig. 1D) (Some precipitants may transform LCP into a sponge phase (Cherezov, et al., 2006). It is fairly difficult to harvest crystals grown in the sponge phase using the described technique. The sponge phase has a liquid-like properties and is drawn away from the well by the surface tension when the well is opened up. To overcome this problem the sponge phase can be transformed back into the cubic phase by lowering the concentration of the precipitant. The cubic phase has a gel-like consistency and stays in place when the well is opened. For example, a common precipitant, PEG 400, at concentrations above 35 %(v/v) can induce sponge phase formation. Lowering PEG 400 concentration to 30 %(v/v) on this step will bring LCP back within few minutes. When lowering PEG 400 concentration keep concentrations of other ingredients unchanged).
- Use an angled sharp needle probe to free one or two edges of the glass square (Fig. 1E) and carefully lift it up (Fig. 1F). The cubic phase bolus can remain on the bottom of the well or can be lifted up with the glass square piece. In the latter case flip the glass piece over and place it inside the well.
- Add an extra 5 μL of precipitant solution, supplemented with a cryo-protectant, if necessary, on top of the exposed cubic phase bolus (Fig. 1G-H) (On this step it is possible to dissolve the lipid and liberate the crystals using an enzymatic lipid digestion (Nollert & Landau, 1998), detergent solutions (Luecke et al., 1999), sponge-inducing compounds (Cherezov et al., 2006) or mineral oils. Any of these treatments, however, could be detrimental to the diffraction quality of the harvested crystals. Typically crystals diffract the best when harvested directly from the cubic phase).
2. Harvesting
- Increase magnification of the microscope to ~100x, focus on a crystal and adjust the polarizer and analyzer angles so that the birefringent crystal has a good contrast with the background, while making sure that there is still enough light to see the harvesting loop.
- Harvest the crystal by scooping it directly from the LCP using a MiTeGen MicroMount with the inner diameter matching the size of the crystal (when harvesting a crystal from the cubic phase, try picking up as little lipid as possible to reduce scattering background from the lipids during data collection. If the crystal is located deep inside the cubic phase bolus, use a micro-tool or an empty MiTeGen MicroMount to remove an excess of the cubic phase from the top and expose the crystal to the surface. Then use a fresh MicroMount to harvest the crystal).
- Immediately plunge the MicroMount with the harvested crystal in a dewar with liquid nitrogen to flash freeze it and then transfer it into a storage or shipping dewar.
For additional details see Caffrey and Cherezov, 2009; Cherezov et al., 2010; Liu and Cherezov, 2011
REFERENCES:
Caffrey, M., and V. Cherezov. (2009) Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4: 706-731. >>
Cherezov, V., Clogston, J., Papiz, M.Z., and M. Caffrey. (2006) Room to move: crystallizing membrane proteins in swollen lipidic mesophases. J. Mol. Biol. 357: 1605-1618. >>
Cherezov, V., Abola, E., Stevens, R.C. (2010) Recent progress in the structure determination of GPCRs, a membrane protein family with high potential as pharmaceutical targets. Methods Mol. Biol. 654: 141-168. >>
Luecke, H., Schobert, B., Richter, H.T., Cartailler, J.P., and J.K. Lanyi. (1999) Structure of bacteriorhodopsin at 1.55 A resolution. J. Mol. Biol. 291: 899-911. >>
Nollert, P., and E.M. Landau. (1998) Enzymic release of crystals from lipidic cubic phases. Biochem. Soc. Trans. 26: 709-713. >>
Liu, W., and Cherezov, V. (2011) Crystallization of membrane proteins in lipidic mesophases. J. Vis. Exp. 49: e2501. >>
Li, D., Boland, C., Aragao, D., Walsh, K., and Caffrey, M. (2012) Harvesting and cryo-cooling crystals of membrane proteins grown in lipidic mesophases for structure determination by macromolecular crystallography. J. Vis. Exp. 67: e4001. >>
CONTACT US: USC | Cherezov Lab | cherezov@usc.edu