
LCP Protocols >> Reconstitution of Protein in LCP
Reconstitution of Protein in LCP
MATERIALS:
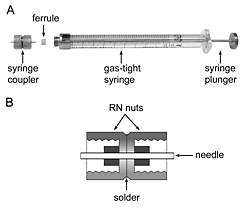
Figure 1. A. Attaching a syringe coupler to a gas-tight syringe. B. A section through the syringe coupler.
- Purified protein solution (Protein solution prepared for crystallization trials should be monodisperse and run as a single sharp peak without significant contribution large oligomeric aggregates on SEC. Protein solution should not contain excessive amounts of detergent, since a high concentration of detergents could destroy the lipidic cubic phase (Ai and Caffrey, 2000; Misquitta and Caffrey, 2003). If detergent creates a problem, try reducing the detergent content as low as possible during purification steps. For example, for His-tagged proteins one can use saturated binding to a Ni sepharose resin and elution with a minimal amount of buffer to concentrate the protein without increasing the detergent content. And finally, use concentrators with the largest possible cutoff. Preferable protein concentration for initial crystallization trials is 10-20 mg/mL).
- Two 100 μL gas-tight removable needle syringes without a needle (Hamilton, cat.# 7656-01).
- 25 μL (Hamilton, cat.# 7654-01) or 50 μL (Hamilton, cat.# 7655-01) gas-tight syringe with a flat tip (point style 3) removable needle (gauge 26s) (Hamilton, cat.# 7768-01).
- Syringe coupler can be made using two gauge 22 removable needles (Hamilton, cat.# 7770-02) and two removable needle (RN) nuts (Hamilton, cat.# 30902) as shown in Fig. 1B and described in Cheng et al., 1998. Alternatively, a similar coupler can be purchased from Formulatrix - SKU 209526, or from Rigaku - cat# EB-LCP-SUNION.
PROCEDURE:
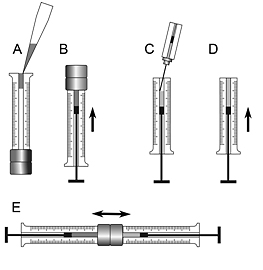
Figure 2. Loading a syringe mixer with lipid and protein and making an LCP..
- Transfer a necessary amount (15 – 50 mg) of a host cubic phase lipid (e.g. monoolein) or a lipid mixture, prepared as described in Lipid Mixing Protocol, into a small 0.5 mL plastic vial.
- Melt lipid at ~40 °C (Melting temperature of monoolein is 37 °C. Different lipids can melt at higher or lower temperature. Adjust the incubation temperature to a few degrees above the lipid melting temperature. Incubation time should be no longer than necessary to melt the lipid, in order to avoid possible degradation).
- Weigh a 100 μL gas-tight syringe with attached coupler (Fig. 1A).
- Remove the plunger and transfer molten lipid into the syringe barrel through the plunger end using an adjustable volume pipette (Fig. 2A) (After taking the molten lipid from incubator it remains liquid at room temperature for a few minutes before it solidifies allowing for transferring it with a pipette).
- Insert the plunger back and slowly move the lipid up in the syringe to remove any trapped air bubbles. Stop when the lipid reaches the end of the coupler needle (Fig. 2B).
- Weigh the syringe to determine the total mass of the lipid in the syringe.
- Use a 25 or 50 μL syringe with a flat tipped 26s gauge needle to transfer an appropriate amount of the protein solution into the second 100 μL gas-tight syringe to achieve 40 wt% of protein solution in the final mixture (for example, for 30 mg of lipid use 20 μL of protein solution) (Fig. 2C). Avoid trapping air bubbles during the syringe loading.
- Move the protein solution with the plunger up as far as possible to minimize the air trapping after assembling the syringe mixer (Fig. 2D).
- Attach the syringe with protein solution to the open end of the coupler mounted on the syringe with lipid.
- Move the protein solution and the lipid through the coupler inner needle back and forth from one syringe to another by pushing alternatively on the corresponding plungers until the lipid mesophase in the syringe mixer become homogeneous and transparent (Fig. 2E). This sequence of motions will mechanically mix the lipid with the protein solution through the action of shearing forces forming inside the narrow coupler needle. Upon mixing a lipidic cubic phase will form spontaneously and the protein will become inserted into the lipid bilayer of the LCP. Complete mixing requires a few hundred passages and typically takes less than 5 minutes (the syringe mixer can warm up due to a friction between the lipidic mesophase and the syringe coupler needle. To avoid heating up the sample, limit the mixing rate to ~1 stroke / s. A useful trick is to slightly cool down the mixer by putting it for a few seconds on ice or in a refrigerator, which speeds up the process of achieving a homogeneous cubic phase. Do not overcool it, however, as below 17°C the monoolein-based LCP is unstable and can convert into a lamellar crystalline phase damaging the protein).
For additional details see Caffrey and Cherezov, 2009.
REFERENCES:
Ai, X., and M. Caffrey. (2000) Membrane protein crystallization in lipidic mesophases: detergent effects. Biophys. J. 79: 394-405. >>
Cheng, A., Hummel, B., Qiu, H., and M. Caffrey. (1998) A simple mechanical mixer for small viscous lipid-containing samples. Chem. Phys. Lipids 95: 11-21. >>
Caffrey, M., and V. Cherezov. (2009) Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4: 706-731. >>
Misquitta, Y, and M. Caffrey. (2003) Detergents destabilize the cubic phase of monoolein: implications for membrane protein crystallization. Biophys. J. 85: 3084-3096. >>
CONTACT US: USC | Cherezov Lab | cherezov@usc.edu